Key milestones for the DNA-prime/protein-boost approach
Dr. Shan Lu was among the first to discover DNA vaccine technology in the early 1990s. It was soon realized that vaccination with DNA alone was not sufficient to elicit strong immune responses in humans with the traditional needle injections. To enhance the immunogenicity of DNA vaccines, Dr. Lu’s group shifted the focus to the development of the DNA-prime/protein-boost approach.
Our first report published in 1998 demonstrated that in rabbits a DNA-prime/protein-boost regimen was more effective than either vaccine component alone in eliciting HIV-1 Env-specific antibodies with high avidity and potent neutralizing activity (Richmond, 1998). While the initial study used Env from lab-adapted strains of HIV, a subsequent collaboration with David Montefiori’s laboratory at the Duke University reproduced this finding with immunogens from a primary HIV-1 clade B isolate JR-FL (Wang, 2005).
In a follow-up rabbit study conducted in collaboration with John Mascola’s laboratory at the Vaccine Research Center, NIH/NIAID, we demonstrated that DNA-prime/protein-boost vaccine with polyvalent Env immunogen mixtures, but not with monovalent Env immunogens, elicited antibodies with neutralizing activities against primary isolates from subtypes A, B, C, D and A/E (Wang, 2006).
Based on the above results, the polyvalent DNA-prime/protein boost vaccine approach was tested in rhesus macaques (Pal, 2006). After immunizations, the animals were challenged rectally with SHIV-Bal virus. While all seven animals in the control group became infected, four out of six vaccinated animals showed no sign of the virus. In the two vaccinated and infected animals, viral load was about 10-fold lower than in the infected control group. Achieving sterilizing immunity demonstrated the promise for a vaccine capable of preventing HIV transmission.
The rhesus macaque study also demonstrated that simple syringe injection of DNA was as efficient at priming immune responses as gene gun DNA delivery. Peak antibody titers after protein boost were similar between the two study groups. This significant finding established the use of traditional needle injection as the delivery approach for DNA vaccines in our platform. This simple approach greatly reduces the cost, time and other resources related to the manufacturing, regulatory review, and the clinical administration of a DNA vaccine, especially for populations in low- and middle-income countries.
Immunological mechanisms of DNA priming
Data accumulated in the last two decades from our group revealed the power of DNA vaccines to prime the immune system for the induction of high magnitude and high quality antibodies in response to recombinant protein immunogens. Several of our studies explored the acquired and innate immune mechanisms that are responsible for this antibody induction role.
First, we observed that DNA priming enhanced the avidity of Env-specific antibody responses in rabbits (Vaine, 2010). Then, in collaboration with Alex Dent at Indiana University, we demonstrated in mice that DNA priming leads to a stronger and more rapid T follicular helper cell and germinal cell responses than priming with proteins (Hollister, 2014). Parallel studies in collaboration with Kate Fitzgerald at the University of Massachusetts Medical School identified the involvement of AIM2 and STING pathways in sensing DNA vaccines and promoting antigen-specific antibody responses (Suschak, 2015; 2016). It became clear from these studies that the combined effects of acquired and innate immune mechanisms activated by DNA vaccines create an environment that strongly promotes subsequent activation and affinity maturation of antigen-specific B cells, leading to high quality antibody responses after a boost with matching protein immunogens.
In summary, our preclinical studies firmly established the utility of two essential elements in our HIV vaccine platform: the use of DNA as the priming immunization step to elicit Env-specific B cells and antibody responses, and the use of a polyvalent Env formulation to expand the breadth of such responses. These principles were at the basis of our first-generation vaccine candidate tested in a Phase I clinical trial DP6-001. Learn more about DP6-001 trial .
The 2nd generation vaccine
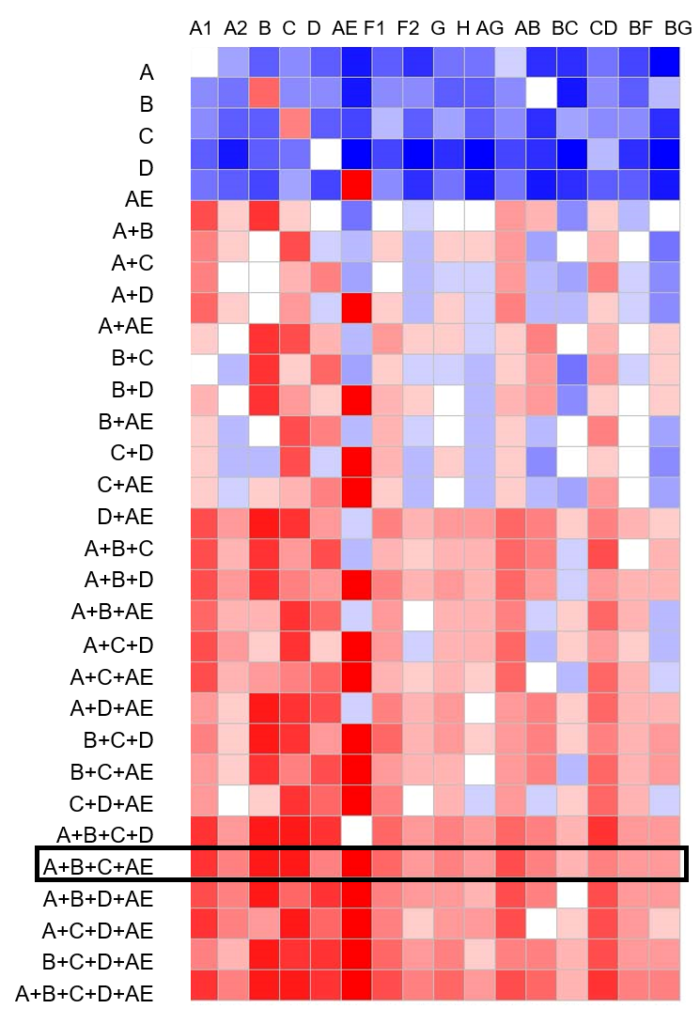
The gp120 immunogens included in the second generation of our vaccine were selected based on a comprehensive screening study, which included more than 60 Env immunogens from primary HIV-1 isolates. Rabbits were primed with DNA expressing each Env from the panel followed by a fixed 5-valent gp120 protein boost. The Envs were ranked based on their ability to prime broad neutralizing antibody responses and top five Env immunogens, each representing one of the five subtypes (A, B, C, D, and A/E), were selected as candidates for a new polyvalent formulation. Our extensive preclinical studies confirmed improved immunogenicity of the new formulation over the original DP6-001 formulation (Wang, 2017).
In order to select the optimal mixture of gp120 immunogens for the next clinical trial, we conducted a bioinformatics analysis predicting the levels of coverage provided by various immunogen combinations against a panel of worldwide HIV-1 Env sequences. The 4-valent mixture of A, B, C, and AE immunogens was predicted to provide over 90% coverage of all 11 major subtypes and recombinant forms of HIV-1 circulating in the world. The results demonstrate the power of polyvalency – a key principle in classical vaccinology. By mixing divergent gp120 immunogens, the coverage of Envs not included in the vaccine was also increased, supporting our hypothesis that a polyvalent vaccine will provide protection against a wide diversity of HIV strains.
Based on these results, we included four gp120 immunogens, one each from clades A, B, C, and AE respectively, into the 2nd generation HIV-1 vaccine, which is currently being tested in a Phase I clinical trial HVTN 124. Learn more about HVTN 124 trial.

