Our first generation vaccine candidate has been tested at the University of Massachusetts Medical School in a phase 1 clinical trial DP6-001. The current, second-generation, candidate PDPHV-201401 was tested in a phase 1a clinical trial HVTN 124 conducted by the NIH-sponsored HIV Vaccine Trials Network (HVTN). The phase 1b trial WHV 138, conducted from 2021 to 2023, further explores the administration of the vaccine components to optimize immune responses. Phase 2a trial WHV 238 is under development and will focus on identifying optimal vaccine formulation and administration schedule to be tested in a Phase 2b efficacy trial. Read more about our trials below.
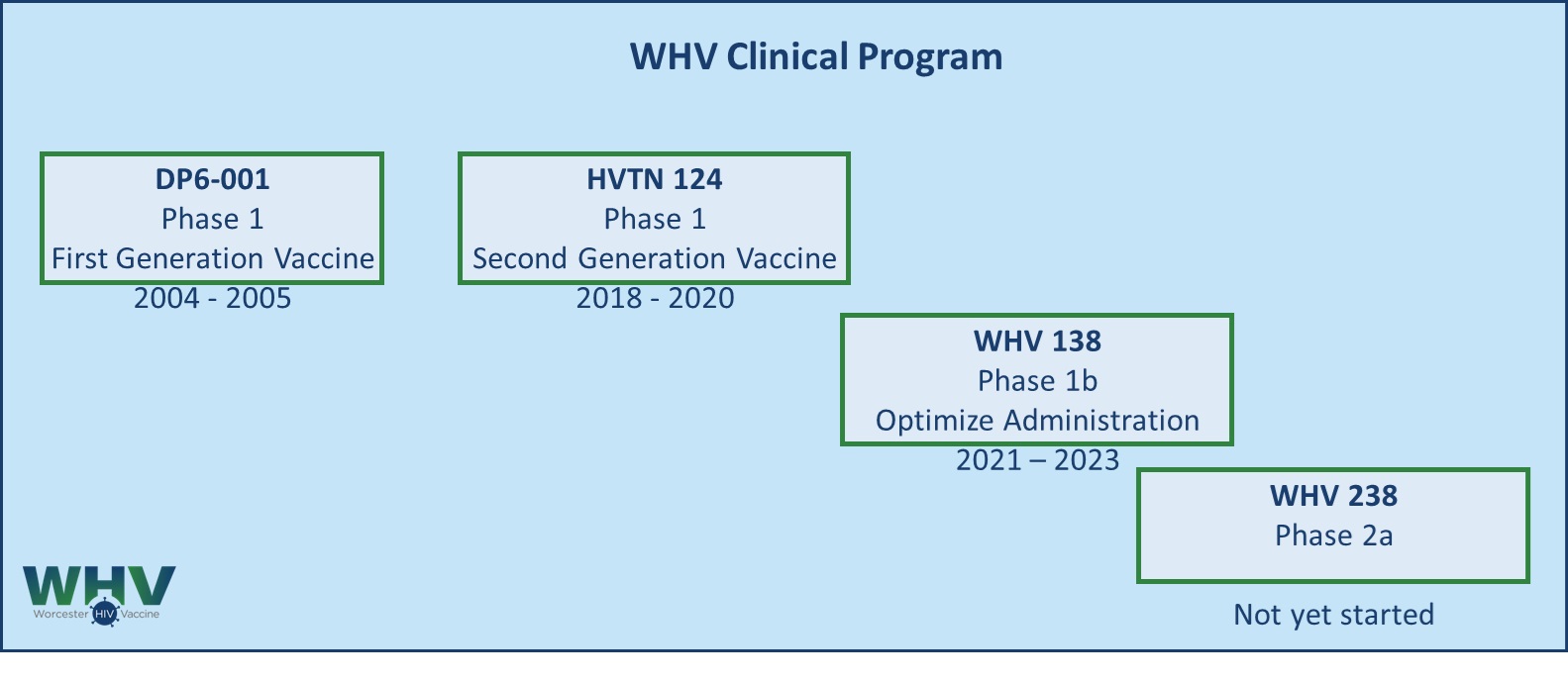
WHV 138 Trial – Phase 1b
Building on the positive results from the HVTN 124 trial, WHV 138 Phase 1b trial explores a simplified administration of the vaccine. Group 1 explores the safety and immunogenicity of DNA and adjuvanted protein administered at different sites for four immunizations over the course of 12 months. Group 2 explores the safety and immunogenicity of the DNA and protein mixed and administered together without the adjuvant for five immunizations over the course of 8 months.
The trial started recruiting in July 2021 and was completed in December 2023.
ClinicalTrials.gov Identifier: NCT04927585
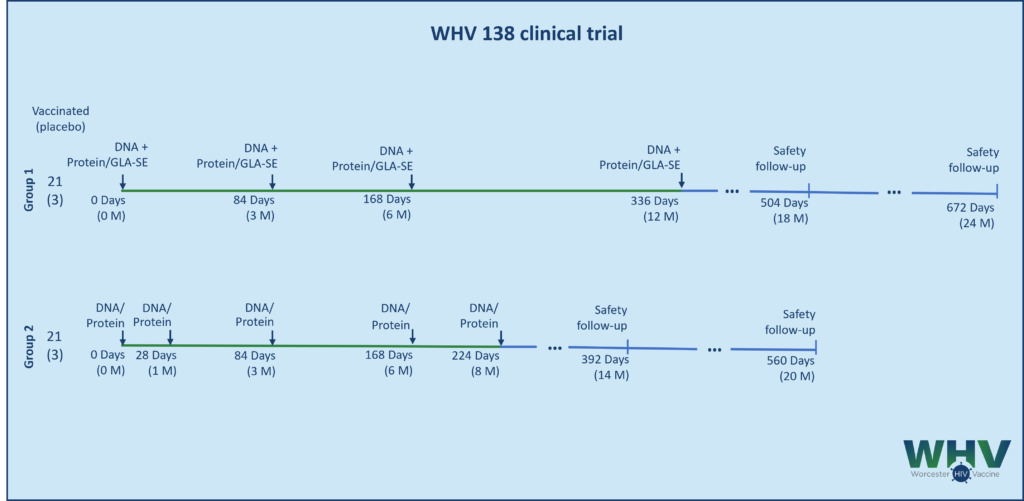
HVTN 124 Trial – Phase 1a
The second generation of the vaccine, PDPHV-201401, was tested in a Phase I trial HVTN 124. The trial explored the safety and the immunogenicity of the vaccine candidate. It also compared the sequential 3 DNA-prime plus 2 protein-boost administration to five simultaneous administrations of DNA and protein at different sites. Learn more about our Vaccine Approach.
HVTN 124 is a Phase I randomized placebo-controlled trial testing the safety and the immunogenicity of WHV’s DNA-prime/protein-boost vaccine candidate. In Part A, volunteers receive two doses of protein to establish safety. After completion of Part A and in the absence of adverse events, Part B of the trial compared sequential and simultaneous administration of DNA and protein components. In both parts, protein is supplemented with GLA-SE adjuvant.
HVTN 124 protocol opened in March, 2018 and was completed in October, 2020. The final clinical study report was published in The Lancet HIV. ClinicalTrials.gov Identifier: NCT03409276
Overall, the PDPHV formulation tested in HVTN124 was well tolerated and both vaccination regimens were found to be safe. More significantly, however, was the immunogenicity of the PDPHV vaccine strategy, which was found to be superior to many previous HIV vaccines in several ways:
- The investigators observed a high response rate (90% to 100%) and high magnitude of titer (>1:10,000) of serum antibody responses among vaccinees against a panel of gp120/p140 antigens from diverse HIV-1 subtypes.
- High level and cross-subtype antibody-dependent cellular cytotoxicity (ADCC) activities were found in most vaccinees as measured by an assay in which the target cells were transfected with infectious molecular clones (IMC). While such assay mimics a viral infection, many of the previous HIV vaccine studies did not elicit ADCC against IMC-transfected target cells.
- Moreover, PDPHV in HVTN 124 induced neutralizing antibody activities against relatively neutralizing resistant Tier 1B viruses across different subtypes (clades B, C, AE and AG) while previous HIV vaccine studies either did not elicit such neutralizing activities or their neutralizing titers were lower than the HVTN 124 sera against the same Tier 1B virus.
- PDPHV elicited high titer IgG responses in 67-100% of participants against gp70-V1V2 antigens from subtypes A, B, C, and AE, an established corelate of protection against HIV infection. The rate and magnitude of these responses exceeded those in previous HIV vaccine studies.
- Furthermore, all volunteers in the prime-boost group achieved 100% CD4+ T cell responses which has not been universally observed in previous human HIV vaccine studies.
DP6-001 Trial – Phase 1 (first generation PDPHV)
The DP6-001 Phase I clinical trial was conducted in 2004-2006 to test the safety and immunogenicity of the first generation of the polyvalent DNA-prime/protein-boost vaccine. This initial formulation was based on the limited number of primary isolates of HIV-1 available in the 1990s and used QS-21 as an adjuvant.
ClinicalTrials.gov Identifier: NCT00061243

Binding antibody titers
In the groups receiving low doses of DNA, most volunteers had undetectable levels of Env-specific antibody responses after 3 DNA immunizations. However, the antibody titers rose quickly after just one protein boost. The anti-gp120 IgG titers reached levels comparable to those observed in chronically infected HIV patients (i.e. 1:105 or higher). By the end of the trial at week 52, participants in group B maintained significant levels of serum anti-gp120 IgG titers. Furthermore, Western blot analysis revealed that antibodies were broadly reactive against a wide range of 11 heterologous primary HIV-1 gp120 antigens (Wang, 2008).

Virus neutralization activity
A study of antibody neutralization activity found high-titer nAb responses in all vaccinated volunteers’ sera against 3 sensitive Tier 1A viruses. Testing the sera against a panel of 11 heterologous primary viruses from subtypes A to E revealed that a majority of volunteers had positive Nab activities against at least half of the pseudotyped viruses expressing primary Env antigens (Wang, 2008). The levels of neutralization were higher than what was reported in previous literature with different HIV vaccine designs that included a DNA component.
ADCC activity
Antibody-dependent cellular cytotoxicity (ADCC) is an important function of antibodies that allows efficient killing of infected cells. A panel of human mAbs was isolated from DP6-001 volunteers and showed potent and broadly reactive ADCC activities (Costa, 2016). In addition, ADCC activities were identified in sera from selected DP6-001 volunteers and the titer increased following each immunization. Further studies are planned to analyze the serum ADCC activities with the entire DP6-001 volunteer population.
T cell immunity
The vaccine also elicited cell-mediated immunity in the volunteers. Overall, 83-100% of volunteers had positive Env-specific IFN-γ responses that were mediated predominantly by polyfunctional CD4+ T cells (Bansal, 2008). HIV-1 specific CD8+ T cell responses were also detected in the high dose group, but at a lower frequency.

